Europe Drug Safety Solutions and Pharmacovigilance Market Demand: Growth, Share, Value, Size, and Insights By 2032
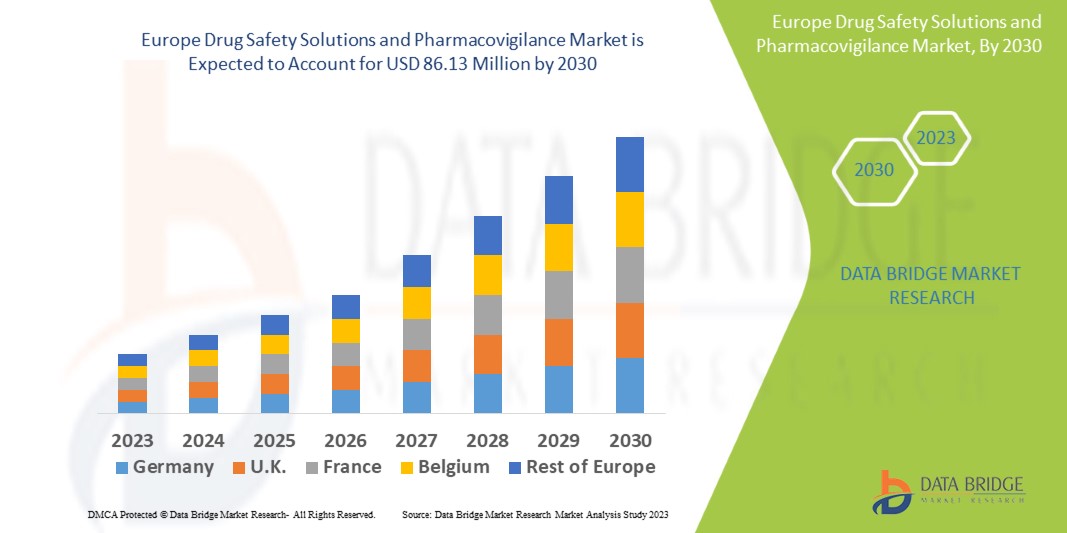
Data Bridge Market Research analyses that the Drug Safety Solutions and Pharmacovigilance s market which was USD 49.75 million in 2022, would rocket up to USD 86.13 million by 2030, and is expected to undergo a CAGR of 7.10% during the forecast period.
Executive SummaryEurope Drug Safety Solutions and Pharmacovigilance Market:
Data Bridge Market Research analyses that the Drug Safety Solutions and Pharmacovigilance s market which was USD 49.75 million in 2022, would rocket up to USD 86.13 million by 2030, and is expected to undergo a CAGR of 7.10% during the forecast period.
An international Europe Drug Safety Solutions and Pharmacovigilance Marketresearch report is an absolute overview of the market that spans various aspects such as product definition, customary vendor landscape, and market segmentation based on various parameters such as type of product, its components, type of management and geography. Most relevant, unique and creditable global market research report is put forth for the valuable customers and clients depending upon their specific business needs. This worldwide market report includes major manufacturers, suppliers, distributors, traders, customers, investors, major types, and major applications. The comprehensive Europe Drug Safety Solutions and Pharmacovigilance Marketbusiness report helps strengthen organization and make better decisions for driving business on the right track.
Europe Drug Safety Solutions and Pharmacovigilance Marketreport assists directing the business in correct direction by giving insights about products, market, customers, competitors and Marketstrategy at exact time. The report introduces top to bottom evaluation of the industry including empowering technologies, key trends, market drivers, challenges, standardization, regulatory landscape, opportunities, future guide, value chain, ecosystem player profiles and strategies. This market research report is a resource that makes available recent as well as upcoming technical and financial details of the industry. A lot of hard work has been involved while generating excellent Europe Drug Safety Solutions and Pharmacovigilance Marketresearch report where no stone is left unturned.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Europe Drug Safety Solutions and Pharmacovigilance Market report. Download Full Report:https://www.databridgemarketresearch.com/reports/europe-drug-safety-solutions-and-pharmacovigilance-market
Europe Drug Safety Solutions and Pharmacovigilance Market Overview
**Segments**
- **By Solution Type**: The Europe drug safety solutions and pharmacovigilance market can be segmented based on solution type into integrated software solutions, autonomous software solutions, and services. Integrated software solutions typically include various features such as adverse event reporting, signal detection, risk management, and compliance monitoring. Autonomous software solutions are becoming increasingly popular due to advancements in technologies like artificial intelligence and machine learning. Services segment includes consulting, outsourcing, and support services, which are essential for ensuring compliance with regulatory requirements and optimizing pharmacovigilance processes.
- **By Deployment Mode**: Another crucial segmentation of the market is by deployment mode, which can be categorized into cloud-based solutions and on-premises solutions. Cloud-based solutions offer flexibility, scalability, and cost-effectiveness, making them attractive to pharmaceutical companies of all sizes. On the other hand, on-premises solutions provide greater control and security over data, which is crucial for organizations with stringent regulatory requirements or data privacy concerns.
- **By End-User**: The Europe drug safety solutions and pharmacovigilance market can also be segmented based on end-users, including pharmaceutical companies, biotechnology companies, contract research organizations (CROs), and other healthcare providers. Each end-user segment has unique needs and challenges when it comes to drug safety and pharmacovigilance, driving demand for tailored solutions and services.
**Market Players**
- **Oracle Corporation**: Oracle offers a comprehensive pharmacovigilance software suite that helps organizations streamline their drug safety processes and comply with regulatory requirements. With features such as adverse event case management, signal detection, and regulatory reporting, Oracle is a key player in the Europe drug safety solutions market.
- **IQVIA**: As a leading provider of healthcare data analytics and technology solutions, IQVIA offers pharmacovigilance services that leverage real-world data to enhance drug safety monitoring and risk management. Their innovative approach to pharmacovigilance makes them a prominent player in the European market.
- **SAS Institute Inc.**: SAS Institute provides advanced analytics and AI-driven solutions for pharmacovigilance, enabling companies to detect potential safety issues and take proactive measures to protect patient health. Their technology-driven approach sets them apart in the competitive landscape of drug safety solutions and pharmacovigilance.
- **Parexel International Corporation**: Parexel offers a range of pharmacovigilance services, including safety case processing, aggregate reporting, and risk management, to support pharmaceutical companies in ensuring the safety and efficacy of their products. With a strong global presence, Parexel is a trusted partner for pharmacovigilance solutions in Europe and beyond.
The Europe drug safety solutions and pharmacovigilance market is witnessing significant growth driven by factors such as increasing regulatory scrutiny, rising demand for real-world data insights, and the emergence of advanced technologies like artificial intelligence and machine learning. One of the key trends shaping the market is the shift towards integrated software solutions that offer a holistic approach to pharmacovigilance, combining features such as adverse event reporting, signal detection, and risk management in a single platform. This trend is driven by the need for streamlined processes and improved efficiency in drug safety monitoring.
Another important trend in the market is the rising adoption of cloud-based solutions among pharmaceutical companies and healthcare providers. Cloud-based solutions offer advantages such as scalability, flexibility, and cost-effectiveness, making them an attractive option for organizations looking to enhance their pharmacovigilance capabilities without significant upfront investments in IT infrastructure. This trend is expected to continue as companies seek more agile and scalable solutions to meet evolving regulatory requirements and market demands.
Furthermore, the increasing focus on real-world evidence and data analytics is driving innovation in pharmacovigilance services. Companies are leveraging advanced analytics and AI-driven technologies to gain deeper insights into drug safety trends, identify potential risks proactively, and improve decision-making processes. This trend is reshaping the pharmacovigilance landscape, enabling companies to move beyond traditional compliance-driven approaches towards more strategic and data-driven risk management practices.
Moreover, the market is witnessing a growing demand for specialized pharmacovigilance services tailored to the needs of different end-users, such as pharmaceutical companies, biotechnology firms, and contract research organizations. These organizations face unique challenges in drug safety monitoring and risk management, requiring customized solutions that address their specific requirements. As a result, market players are focusing on developing niche offerings and partnerships to cater to the diverse needs of the industry stakeholders effectively.
In conclusion, the Europe drug safety solutions and pharmacovigilance market is evolving rapidly, driven by technological advancements, regulatory changes, and shifting market dynamics. Companies that can innovate, collaborate, and adapt to these trends are well-positioned to capitalize on the growing opportunities in this dynamic and competitive market landscape. The future of pharmacovigilance lies in leveraging advanced technologies, real-world data insights, and tailored solutions to enhance patient safety, improve regulatory compliance, and drive better health outcomes for individuals worldwide.The Europe drug safety solutions and pharmacovigilance market is a dynamic and competitive landscape driven by various factors such as regulatory scrutiny, technological advancements, and evolving market demands. One key aspect shaping the market is the trend towards integrated software solutions that offer a comprehensive approach to pharmacovigilance processes. These solutions combine functionalities like adverse event reporting, signal detection, and risk management in a single platform, enabling organizations to streamline their drug safety activities and enhance operational efficiency. The demand for such integrated solutions is driven by the need for a more holistic and centralized approach to pharmacovigilance, ensuring compliance with regulatory requirements and improving risk management practices.
Another significant trend in the market is the increasing adoption of cloud-based solutions by pharmaceutical companies and healthcare providers. Cloud-based solutions offer benefits such as scalability, flexibility, and cost-effectiveness, making them an attractive option for organizations looking to enhance their pharmacovigilance capabilities without substantial upfront investments. The scalability and agility provided by cloud solutions enable companies to adapt to evolving regulatory requirements and market dynamics more effectively, driving their competitiveness in the market.
Furthermore, the trend towards leveraging real-world data insights and advanced analytics in pharmacovigilance services is reshaping the industry landscape. Companies are increasingly utilizing AI-driven technologies to analyze vast amounts of data and identify potential safety issues proactively, leading to more informed decision-making and improved patient outcomes. This shift towards data-driven risk management practices is enabling organizations to move beyond traditional compliance-driven approaches and embrace more strategic and proactive pharmacovigilance strategies.
Moreover, the market is witnessing a growing demand for specialized pharmacovigilance services tailored to the unique needs of different end-users such as pharmaceutical companies, biotechnology firms, and CROs. These organizations face distinct challenges in drug safety monitoring and risk management, necessitating customized solutions to address their specific requirements effectively. Market players are focusing on developing niche offerings and forming strategic partnerships to cater to the diverse needs of industry stakeholders and capitalize on the evolving market opportunities.
In conclusion, the Europe drug safety solutions and pharmacovigilance market is undergoing rapid transformation driven by technological innovations, regulatory changes, and evolving market trends. Companies that can adapt to these changing dynamics by embracing integrated software solutions, cloud-based technologies, data-driven analytics, and specialized services tailored to end-users' needs will be well-positioned to succeed in this competitive market landscape. The future of pharmacovigilance lies in harnessing advanced technologies, leveraging real-world data insights, and collaborating with industry stakeholders to enhance patient safety, ensure regulatory compliance, and improve overall health outcomes globally.
The Europe Drug Safety Solutions and Pharmacovigilance Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now:https://www.databridgemarketresearch.com/reports/europe-drug-safety-solutions-and-pharmacovigilance-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
What insights readers can gather from theEurope Drug Safety Solutions and Pharmacovigilance Marketreport?
- Learn the behavior pattern of everyEurope Drug Safety Solutions and Pharmacovigilance Market-product launches, expansions, collaborations and acquisitions in the market currently.
- Examine and study the progress outlook of the global Europe Drug Safety Solutions and Pharmacovigilance Marketlandscape, which includes, revenue, production & consumption and historical & forecast.
- Understand important drivers, restraints, opportunities and trends (DROT Analysis).
- Important trends, such as carbon footprint, R&D developments, prototype technologies, and globalization.
Browse More Reports:
Global Oryzanol Market
Global Viral Conjunctivitis Market
Global Aneurysm Clips Market
Europe Shipping Container Liner Market
Global Agricultural Lubricants Market
Global Concrete Floor Coatings Market
Global Influenza Testing Market
Global Refsum Disease Market
Global Panthenol Market
Europe Charge-Coupled Device (CCD) Imagers Market
North America Wood Based Panel Market
Middle East and Africa Gloves Market
Global Smart Window Market
Global Microbial Agricultural Inoculants Market
Global Inverted Pouches Market
Global Spinal Machined Bone Allograft Market
Global Unified Communication as a Service (Ucaas) Market
Global Microscopy-Based RNA Imaging Techniques Market
Global Pharmaceutical Logistics Market
U.S. Glass Door Merchandiser Market
Global Hoses Market
U.S. Private Label Food and Beverages Market
Global Plastic Ready Meal Trays Market
Global Industrial Absorbents Market
Global Fuel Cells Marine Vessels Market
Global Pleurisy Market
Global Medical Nitrile Gloves Market
Global Sheet Metal Market
Global Fortified Beverages Market
North America Charge-Coupled Device (CCD) Imagers Market
Global Scintillators Market
Europe Cocoa Products Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:-corporatesales@databridgemarketresearch.com




























